MPCT-021N | Systemic Lupus Erythematosus (SLE) with or without Lupus Nephritis (LN)

Learn more about a clinical study investigating a new potential treatment option for people living with Systemic Lupus Erythematosus (SLE) with or without Lupus Nephritis (LN)
Click here to learn more about each section below:

What is the MPCT-021N Study?
Who can join the MPCT-021N study?
People may be able to join the study if they meet the following requirements*:
*Other study requirements will apply
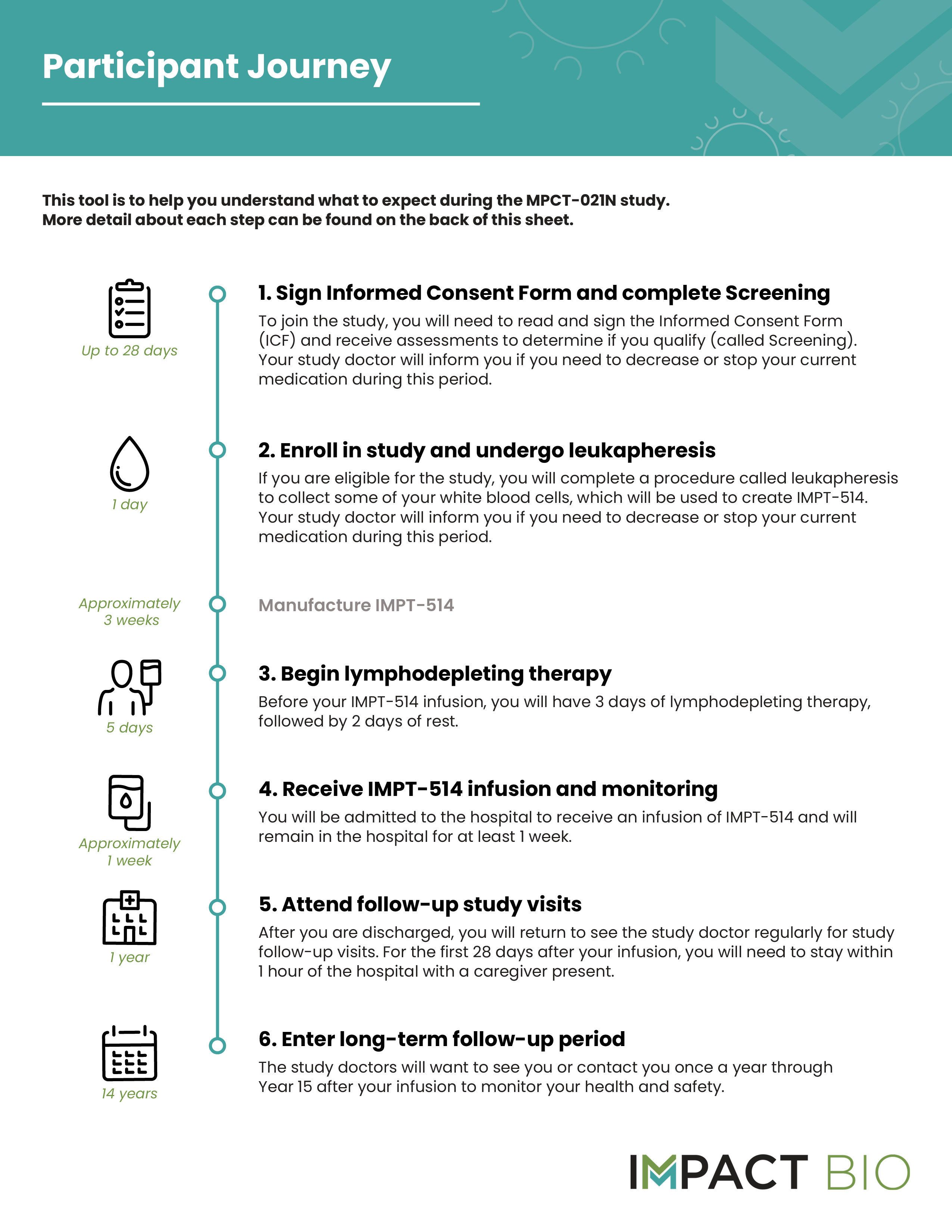
What will happen during the study?
Participation in the MPCT-021N study includes a main treatment and follow up period that lasts about 1 year, as well as a long-term safety follow up period that extends to 15 years. The FDA requires that participants receiving cell therapy products such as IMPT-514 are followed for up to 15 years for long term safety monitoring. Study participants can expect the following:
Click to download a printable MPCT-021N Study Participant Journey PDF here:
Participation in a clinical study is voluntary. You can ask any questions you have and may leave the study at any time, for any reason.

About IMPT-514
What is the study drug?
IMPT-514 is a type of CAR T-cell therapy. To create IMPT-514, some of your own white blood cells will be collected and modified so that they can identify the B cells that are causing inflammation. Then, the white blood cells will be given back to you by intravenous infusion to potentially kill the B cells that are causing the inflammation. IMPT-514 is investigational, which means it can only be used in research studies. It has not been approved by the United States Food and Drug Administration (FDA) as a therapy for lupus.
Will I receive the study drug?
Yes, as a participant in the MPCT-021N study, you will receive active study treatment.
What is an “investigational drug?”
Investigational means the study treatment is not approved by regulatory authorities like the US Food and Drug Administration (FDA), and it can only be used in clinical research studies like MPCT-021N.
About Systemic Lupus Erythematosus (SLE) with or without proliferative Lupus Nephritis (LN)
What is Systemic Lupus Erythematosus (SLE) with or without proliferative Lupus Nephritis (LN)?
Systemic Lupus Erythematosus (SLE) with or without Lupus Nephritis (LN) is a chronic autoimmune disease characterized by widespread inflammation that may involve multiple organ systems (most commonly the skin and joints) and significantly impact patient quality of life. Lupus Nephritis is a type of SLE with significant inflammation in the kidneys. Over time, such kidney inflammation can lead to kidney failure.
Where can I learn more?
If you have additional questions about participating in this clinical research study, contact a study clinic near you. Travel support may be available, if needed. To see a full list of all participating sites in this trial please click here to visit the MPCT-021N study page on www.clinicaltrials.gov

Overview of Clinical Trials
Here are some common questions and answers about study participation.
What are clinical research studies (trials)?
Clinical research studies, or trials, help scientists and doctors explore whether a medical strategy, device, or medication is safe and effective for people. Before any medication can be approved and made available to the public, it must go through several phases of clinical research.
What is informed consent?
Before enrolling in a clinical trial, you must sign an Informed Consent Form (ICF). The ICF contains information about the study, including study goals, how long the study will last, benefits and risks, and the tests and procedures you will receive.
What does study participation involve?
Study participation usually involves visiting a clinic regularly, taking or receiving an investigational medicine, and having assessments to monitor your health. You can still see your regular doctor, but you should let them know that you are participating in a study.
Participation in clinical research studies is your choice, and you may stop at any time.
Click here to find out more about our company on our LinkedIn page: