ImmPACT Bio Announces FDA Clearance of IND Application for CD19/CD20 Bispecific CAR-T Cell Therapy in Multiple Sclerosis
ImmPACT Bio Announces FDA Clearance of IND Application for CD19/CD20 Bispecific CAR-T Cell Therapy in Multiple Sclerosis IMPT-514 is the first-and-only known CD19/CD20 CAR T-cell therapy in development for multiple sclerosis Dual CD19/CD20 targeting is designed to enable broad depletion of pathogenic autoimmune cells Initiation of Phase 1 dose exploration trial expected first half of […]
ImmPACT Bio Appoints Ian Somaiya to its Board of Directors

LOS ANGELES, August 14, 2024 – ImmPACT Bio USA Inc. (ImmPACT Bio), a clinical-stage biopharmaceutical company developing a new generation of cellular therapies that have the potential to bring transformational benefits to patients, today announced the appointment of Ian Somaiya to its board of directors. Mr. Somaiya, currently chief financial officer (CFO) of NewAmsterdam Pharma Company […]
Sylvain Roy, CTO to present at Cell and Gene Therapy Manufacturing and Commercialization

Sylvain Roy, CTO to prsent at Cell and Gene Therapy Manufacturing and Commercialization September 23-26, 2024 Hynes Convention Center, Boston, MA https://informaconnect.com/cell-therapy-bioprocessing/speakers/sylvain-roy/
Manufacturing of CD19/20 Bispecific CAR T-Cell Therapy (IMPT-514) for the Treatment of Multiple Sclerosis
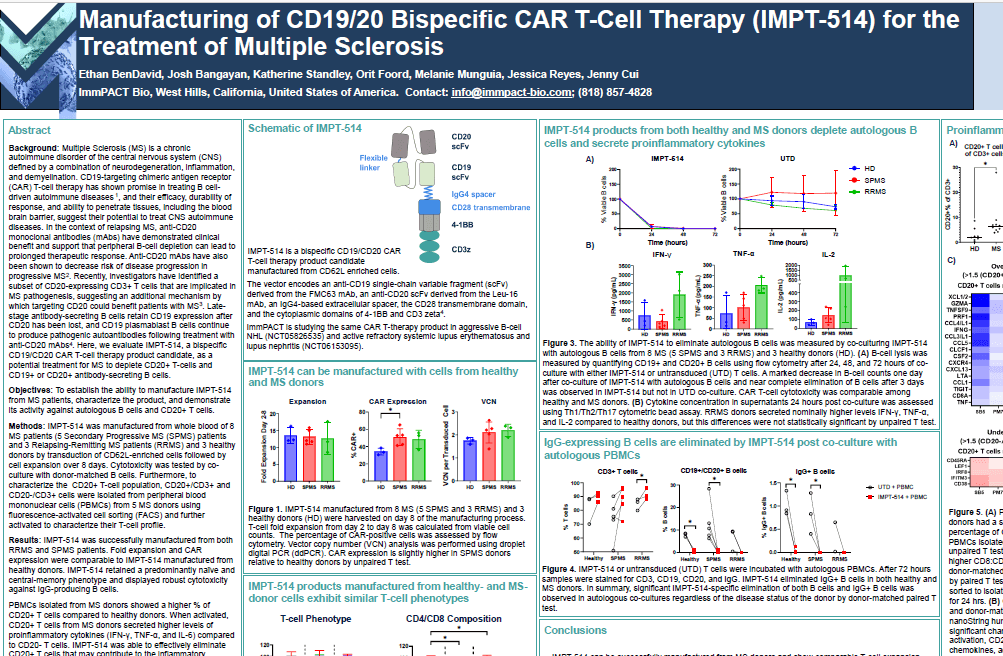
Ethan BenDavid, Josh Bangayan, Katherine Standley, Orit Foord, Melanie Munguia, Jessica Reyes, Jenny Cui poster presentation at the Consortium of Multiple Sclerosis (CMSC) Annual Meeting May 30, 2024. View PDF
Engineering a Bispecific Claudin 18.2/TGF-β CAR to Target Claudin 18.2 Tumors and Overcome the Tumor Microenvironment
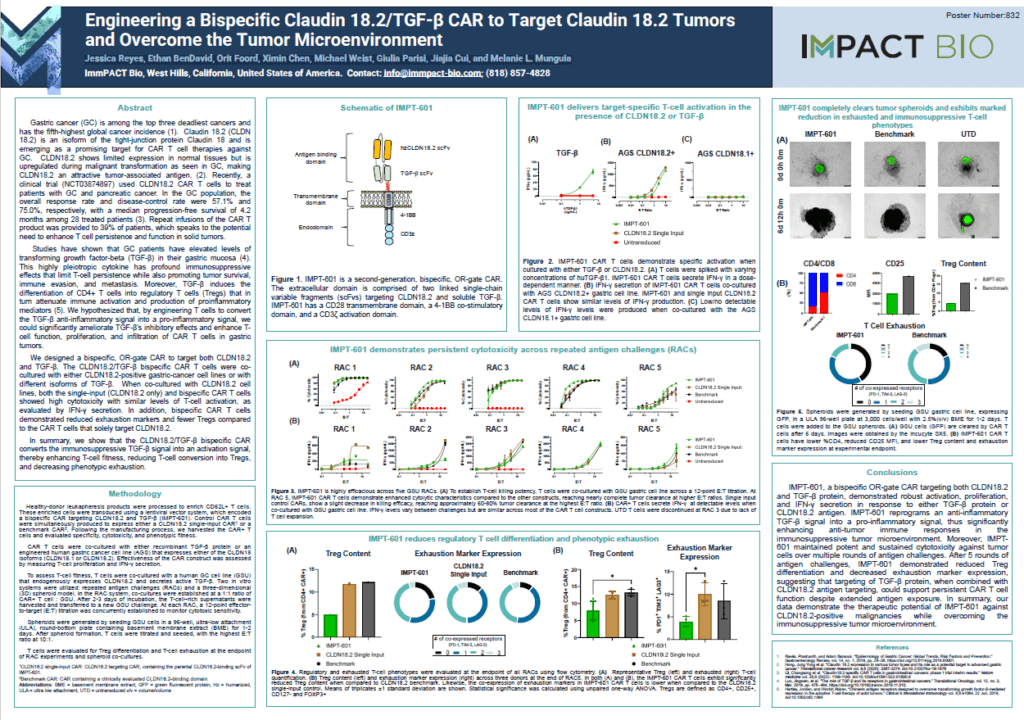
Jessica Reyes, Ethan BenDavid, Orit Foord, Ximin Chen, Michael Weist, Giulia Parisi, Jiajia Cui, and Melanie L. Munguia poster presentation at the American Society of Gene and Cell Therapy (ASGCT) 27th Annual Meeting, May 8, 2024, in Baltimore, Maryland. View PDF
